About our HACCP Training Courses
HACCP Training Courses Comparison
| HACCP Introduction/Refresher Certificate | HACCP Introduction/Refresher Certificate | Advanced HACCP Certificate |
|---|---|---|
| Duration: 1 Day | Duration: 1 Day | Duration: 2 Days |
| For: All Food Sectors | For: All Food Sectors | For: All Food Sectors |
| Suitable for: Operators | Suitable for: Supervisors | Suitable for: Senior Supervisors/HACCP Review Team Members |
| Global standardization:
Yes, standardized to Global Codex Alimentarius & GFSI |
Global standardization:
Yes, standardized to Global Codex Alimentarius & GFSI |
Global standardization:
Yes, standardized to Global Codex Alimentarius & GFSI |
| Standards comparable with other countries: | Standards comparable with other countries:
Yes, FDA Comparability Read Report |
Standards comparable with other countries:
Yes, FDA Comparability Read Report |
| Course Content: | Course Content: | Course Content: |
|
|
|
|
|
|
|
|
|
|
|
|
| Focus: Operator Monitoring & Verification, Corrective Action skills | Focus: Operator/Supervisor Monitoring & Verification, Corrective Action, Advanced RCA skills | Focus: Senior Supervisor/QA/Technical/Management: All + HACCP Plan Dev. + Review skills |
| Achievement:
HACCP Training Certificate/HACCP Refresher Certificate (if already trained) |
Achievement:
HACCP Training Certificate/HACCP Refresher Certificate (if already trained) |
Achievement:
Advanced HACCP Training Certificate |
| Who can achieve a certificate?
New Zealand & All Other Countries |
Who can achieve a certificate?
New Zealand & All Other Countries |
Who can achieve a certificate?
New Zealand & All Other Countries |
| Additional option: Only for currently employed people working in New Zealand Food Companies | Additional option: Only for currently employed people working in New Zealand Food Companies | Additional option: Only for currently employed people working in New Zealand Food Companies |
| Additional cost?
Yes (for on-job assessor time + NZQA Admin.) |
Additional cost?
Yes (for on-job assessor time + NZQA Admin.) |
Additional cost?
Yes (for on-job assessor time + NZQA Admin.) |
| HACCP Training Courses NZ | HACCP Training Courses NZ | HACCP Training Courses NZ |
|
|
|
| Requires: On-job verification (application of learning back on-job) | Requires: On-job verification (application of learning back on-job) | Requires: On-job verification (application of learning back on-job) |
Here’s an introduction to HACCP which stands for Hazard Analysis Critical Control Point.
Our course contents:
- Case study review of recent food safety breakdowns and learning.
- Introduction to legislative requirements for HACCP plans and their importance
- Supporting cGMP eg. Training requirements, pest control, Illness management, Hygiene, Cleaning & Sanitising
- Introduction to the 7 Principles of HACCP
- Introduction to food safety hazards (Physical / foreign matter-P; Chemical & Allergenic-C, Microbiological-M Pathogenic bacteria, toxins, moulds and yeast) – Simplified, detailed and VISUAL
- HACCP as a tool for identifying and controlling potential hazards Critical Control Points (CCPs) and critical limits relevant to the workplace and department requirements
- Corrective actions to be taken if limits are exceeded. Codex 3 step CA methodology.
- Record keeping – Food Control Plans and RMPs Visual application of HACCP with videos and practical examples
Applying HACCP – Part 1
The video below explains how HACCP is used to make products safe
Codex Alimentarius Definitions & Useful Information:
Food Safety Hazard
A biological, chemical or physical agent in, or condition of, food with the potential to cause an adverse health effect.
What should the hazard analysis identify?
Involves identification & analysis of hazards reasonably likely to occur associated with each input and each process step. The analysis should identify those hazards that are occurring at an unacceptable level, possible control measures.
The meaning of ‘reasonably likely to occur’
‘Reasonably likely to occur’ means that the particular hazard is known to occur in the particular food ingredient being used or food being produced in New Zealand.
What sources of information can we use to determine the hazard is RLO?
This knowledge is based on scientific reports, industry or company results, Codes of Practice, and information from the regulator (e.g. hazard database).
What is the purpose of the analysis, and what is the relationship of control measures to the hazard analysis?
The purpose of the analysis will establish which hazards are at unacceptable levels and control measures available for controlling the hazard.
What are the options for the management of hazards?
This could include control under Good Operating Practice, by a CCP or the fact that no control measures are available.
What is the Codex definition of a critical control point (CCP)?
A step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level.
What tool can be utilised to facilitate CCP determination?
Determination can be facilitated by use of a decision tree or a table but the candidate must understand and demonstrate how it works.
Define the meaning of ‘acceptable level’ and how it is determined, include definitions of regulatory limit (RL) and operator-defined limit (ODL) and provide at least one example of a RL and an ODL
The acceptable level is a level of a specific hazard considered acceptable for food safety. Provided by a: Regulatory limit (RL) for a specific hazard, or Operator-defined limit (ODL) for a specific hazard.
A regulatory limit (RL) is a measurable regulatory requirement that is critical to the fitness for intended purpose of a product. Examples of RLs for food safety: 0cfu/25gm listeria monocytogenes in ready-to-eat food (not supporting L.m). 3.78 log10CFU campylobacter/poultry carcass (Schedule 1, NMD programme).
An operator-defined limit (ODL) is a measurable limit that is established by the food business operator to manage the fitness for intended purpose of a product. The limit may be essential for food safety but is not set in legislation. Examples of ODLs are: 12 D reduction of clostridium botulinum in low-acid canned food. Pasteurisation at 72°C for 15 seconds.
What action would be taken if no control measure exists for the identified hazards?
If no control measure exists and a hazard has been identified at a step where control is necessary for food safety, then product or process re-design should be considered.
Can there be more than one hazard controlled by a single CCP or a single CCP controlling multiple hazards?
Yes, may be more than one CCP to address same hazard. Yes, maybe one CCP to address one or more hazards.
What is the Codex definition of a critical limit? Give at least one example of a critical limit?
A criterion which separates acceptability from unacceptability.
Examples are time, temperature, pH, water activity. Describe key features of critical limits, the requirements that need to be met for the establishment of a critical limit. CLs must be achievable, measurable, justifiable; must allow for monitoring and corrective action to be taken quickly. The CL(s) must be specified for each CCP and be validated.
What is the Codex definition of monitoring?
The scheduled measurement or observation of a CCP relative to its CLs.
What is being monitored, and what is monitoring expected to achieve?
The monitoring must be able to detect loss of control at the CCP. Monitoring involves measurements/observations that can be done quickly to detect deviations and allow the operator to regain control immediately. Monitoring includes: who, what, when, where, and how.
What is the difference between 100% monitoring and other approaches and what are the strengths and weaknesses of each approach?
The candidate needs to understand what the difference is between continual monitoring and monitoring by sampling plan and the strengths and weaknesses of each. Particularly in relation to product disposition when things go wrong, i.e. part of taking corrective action. If monitoring is not continuous then the amount or frequency of monitoring must be sufficient to guarantee that the CCP is in control.
What is the Codex definition of corrective action?
Action to be taken when the results of monitoring at the CCP indicate a loss of control (i.e. CLs not met).
What does corrective action include?
Corrective action includes: Restoration of control Proper disposition of non-complying products Prevention of re-occurrence.
What is the Codex definition of verification and what does it include?
Application of methods, procedures, tests and other checks in addition to monitoring, to determine compliance with the documented HACCP application. Verification includes: Ongoing review Checks when there are significant changes to the products or processes Audit Reality checks (e.g. checking the checker) A check of records generated. Includes who, what, when, where, and how.
How is the frequency of verification determined?
Frequency should be sufficient to confirm that the HACCP system is working effectively and is based on factors such as performance, new processes and people and the like.
What is the most recent (2008) Codex definition of validation?
Validation: Obtaining evidence that a control measure or a combination of control measures, if properly implemented, is capable of controlling the hazard to a specified outcome (Codex 2008)
What is the Codex principle relating to documentation and record-keeping?
Includes documentation of all HACCP principles applied (HACCP procedures) and the generation of records for CCP monitoring, corrective action, verification (including validation).
Define what documentation is kept and why?
Documentation is required for the scope, product and process description and seven HACCP principles as relevant to the HACCP application. Uses of documentation include verification, training staff, and demonstrating that there is a programme to manage hazards appropriately.
Records of CCP monitoring, corrective action and verification (including validation) are needed to provide evidence that the system has been implemented effectively and that any problems have been corrected in an appropriate manner.
Other ISO definitions
Food chain
The sequence of the stages and operations involved in the production, processing, distribution, storage and handling of a food and its ingredients, from primary production to consumption
NOTE 1 This includes the production of feed for food-producing animals and for animals intended for food production.
NOTE 2 The food chain also includes the production of materials intended to come into contact with food or raw materials.
Food safety policy
Overall intentions and direction of an organization related to food safety as formally expressed by top management
End product
Product that will undergo no further processing or transformation by the organization
NOTE A product that undergoes further processing or transformation by another organization is an end product in the context of the first organization and raw material or an ingredient in the context of the second organization.
Flow diagram
Schematic and systematic presentation of the sequence and interactions of steps
PRP ( prerequisite programme) 〈food safety〉
Basic conditions and activities that are necessary to maintain a hygienic environment throughout the food chain suitable for the production, handling and provision of safe end products and safe food for human consumption
NOTE The PRPs needed to depend on the segment of the food chain in which the organization operates and the type of organization Examples of equivalent terms are Good Agricultural Practice (GAP), Good Veterinary Practice (GVP), Good Manufacturing Practice (GMP), Good Hygienic Practice (GHP), Good Production Practice (GPP), Good Distribution Practice (GDP) and Good Trading Practice (GTP).
OPRP (Operational prerequisite programme)
Identified by the hazard analysis as essential in order to control the likelihood of introducing food safety hazards to and/or the contamination or proliferation of food safety hazards in the product(s) or in the processing environment
Updating Immediate and/or planned activity to ensure application of the most recent information Definitions related to PRPs / GMP / SOPs Contamination 〈food safety〉 Introduction or occurrence of a contaminant in food or food environment NOTE Adapted from CAC/RCP 1:2003, 2.3.
Contaminant 〈food safety〉
Any biological or chemical agent, foreign matter or other substances not intentionally added to food which may compromise food safety or suitability [CAC/RCP 1:2003, 2.3]
Establishment 〈food safety〉
Any building or area in which food is handled and the surroundings under the control of the same management [CAC/RCP 1:2003, 2.3]
Materials 〈food safety〉
General term used to indicate raw materials, packaging materials, ingredients, process aids, cleaning materials and lubricants
Cleaning 〈food safety〉
Removal of soil, food residue, dirt, grease or other objectionable matter NOTE Adapted from CAC/RCP 1:2003, 2.3. Product contact All surfaces that are in contact with the product or the primary package during normal operation
Material specification / product specification 〈food safety〉
Detailed documented description or enumeration of parameters, including permissible variations and tolerances, which are required to achieve a defined level of acceptability or quality Food-grade Lubricants and heat transfer fluids formulated to be suitable for use in food processes where there may be incidental contact between the lubricant and the food
Disinfection 〈food safety〉
Reduction, by means of chemical agents and/or physical methods, of the number of microorganisms in the environment, to a level that does not compromise food safety or suitability NOTE Adapted from CAC/RCP 1:2003[1], 2.3.
Cleaning in place CIP Cleaning (of equipment by impingement or circulation of flowing chemical solutions, cleaning liquids and water rinses into, on to and over surfaces in equipment or systems without dismantling and designed for the purpose
Cleaning out of place COP System where equipment is disassembled and cleaned in a tank or in an automatic washer by circulating a cleaning solution and maintaining a minimum temperature throughout the cleaning cycle
Sanitizing 〈food safety〉
Process of cleaning, followed by disinfection Sanitation All actions dealing with cleaning or maintaining hygienic conditions in an establishment, ranging from cleaning and/or sanitizing of specific equipment to periodic cleaning activities throughout the establishment (including building, structural, and grounds cleaning activities)
Certificate of analysis COA 〈food safety〉
Document provided by the supplier which indicates results of specific tests or analysis, including test methodology, performed on a defined lot of the supplier’s product Zoning 〈food safety〉 Demarcation of an area within an establishment where specific operating, hygiene or other practices may be applied to minimize the potential for microbiological cross-contamination
NOTE Examples of practices include: clothing change on entry or exit, positive air pressure, modified traffic flow patterns.
Applying HACCP – Part 2
The video below explains how HACCP is technically applied to build a HACCP Plan
Advanced HACCP Training Course Content
HACCP Team (5)
The food operation should assure that the appropriate product-specific knowledge and expertise is available for the development of an effective HACCP Plan. Optimally, this may be accomplished by assembling a multidisciplinary team. Where such expertise is not available on site, expert advice should be obtained from other sources. Scope of HACCP Plan
(6) The scope of the HACCP Plan should be identified.
The scope should describe which segment of the food chain is involved and the general classes of hazards to be addressed (e.g. does it cover all classes of hazards or only selected classes?). Describe Product
(7) A full description of the product should be drawn up, including relevant safety information such as: composition, physical/chemical structure (including Aw, pH, etc.), microcidal/static treatments (heat-treatment, freezing, brining, smoking, etc.), packaging, durability and storage conditions and method of distribution. Identify Intended Use/Intended Consumer
(8) The intended use should be based on the expected uses of the product by the end user or consumer. In specific cases, vulnerable groups of the population, e.g. institutional feeding, may have to be considered.
(9) The description of the intended use should identify, where appropriate: a. Normal usage conditions, e.g. appropriate storage temperatures, and how it is likely to be eaten b. Potential for abuse of the product, e.g. the likelihood of incorrect storage or handling of the product, resulting in unacceptable growth of microorganisms. Product Safety Outcomes
(10) The HACCP team must determine what the organisation intends to achieve in terms of product safety outcomes for each product. Construct Flow Diagram
(11) The flow diagram should be constructed by the HACCP team. The flow diagram should cover all steps in the operation. When applying HACCP to a given operation, consideration should be given to steps preceding and following the specified operation.
(12) The inputs must be described. These include raw materials, ingredients, food additives, and wrapping and packaging materials or containers that come into direct contact with or form part of the product, e.g. plastic bag liners etc.
(13) Edible outputs should also be shown. Each of these may initiate a separate process flow diagram of its own and form part of another HACCP Plan with a different end product.
(14) The flow diagram should include all activities which impact on the process which has been scoped e.g. reworking etc. Where trials have the potential to impact on mainstream processes, the impact should also be subject to a hazard analysis. On-site Confirmation of Flow Diagram
(15) The HACCP team should confirm the processing operation against the flow diagram during all stages and hours of operation and amend the flow diagram where appropriate.
(16) It is important that the process flow diagram reflects what is actually happening with the process. On completion, the process flow diagram should be confirmed. Hazard Identification, Hazard Analysis and Control Measures
(17) List all potential hazards associated with each step, conduct a hazard analysis, and consider any measures to control identified hazards (SEE PRINCIPLE 1).
List All Potential Hazards
(18) The HACCP team should list all of the hazards that may be reasonably expected to occur at each step from primary production, processing, manufacture, and distribution until the point of consumption.
(19) All biological, chemical and physical hazards should be considered.
(20) Identify the hazard source and be specific in terms of the actual hazard. The level of specificity is determined by the extent of hazard identification required to ensure effective hazard control. (e.g. for raw milk instead of writing “biological hazard” be more specific and write “pathogenic bacteria, such as E. coli, Listeria spp, Salmonella spp, Staphylococcus aureus”). This will ensure that the control measures are relevant and effective in controlling the specific hazard(s) identified.
Conduct a Hazard Analysis
(21) The HACCP team should next conduct a hazard analysis to identify for the HACCP Plan, which hazards are of such a nature that their elimination or reduction to acceptable levels is essential to the production of a safe food. In conducting the hazard analysis, wherever possible the following should be included:
(a) the likely occurrence of hazards and severity of their adverse health effects; (see footnote) { The likely occurrence of hazards and severity of their adverse health effects gives the probability of occurrence and severity of illness};
(b) the qualitative and/or quantitative evaluation of the presence of hazards;
(c) survival or multiplication of microorganisms of concern;
(d)production or persistence in foods of toxins, chemicals or physical agents; and
(e) conditions leading to the above.
(22) Analyze each hazard and decide which are significant in relation to the designated product outcome. A significant hazard is one that is likely to occur at unacceptable levels.
Control Measures
(23) The HACCP team must then consider what control measures, if any, exist which can be applied for each hazard. More than one control measure may be required to control a specific hazard(s) and more than one hazard may be controlled by a specified control measure.
(24) For each hazard identified as reasonably likely to occur, list the control measures or prerequisite programmes that are in place. Control measures are specific to the process step where the hazard has been identified whereas prerequisite programmes control hazards that could come into contact with product and are more generic in nature.
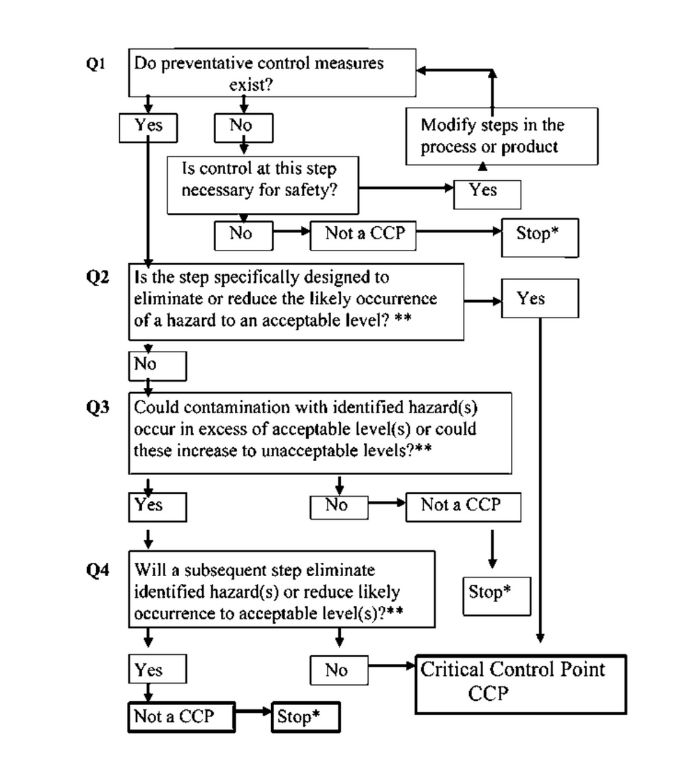
(25) A control measure/prerequisite programme must be effective in controlling the specific hazard(s) identified, implemented and working on a consistent basis. Where control is not effective the HACCP team should determine the gaps and implement controls as necessary. Determine Critical Control Points (SEE PRINCIPLE 2)
(26) There may be more than one CCP at which control is applied to address the same hazard. The determination of a CCP in the HACCP system can be facilitated by the application of a decision tree [e.g. Figure A1.1], which indicates a logic reasoning approach. Application of a decision tree should be flexible, given whether the operation is for production, slaughter, processing, storage, distribution or other. It should be used for guidance when determining CCPs. This example of a decision all potential hazards that are reasonably likely to occur have been identified, tree may not be applicable to all situations. Other approaches may be used. Training in the application of the decision tree is recommended.
(27) If a hazard has been identified at a step where control is necessary for safety, and no control measure exists at that step, or any other, then the product or process should be modified at that step, or at any earlier or later stage, to include a control measure.
(28) CCPs can be identified by means other than the Codex Decision Tree, as long as meaningful analysis of each identified significant hazard is undertaken in relation to expected product outcomes for the product.
(29) The existence or non-existence of a CCP should never be assumed without working through some systematic decision-making process.
Figure A1.1: Codex Alimentarius Decision Tree to Identify CCPs
Establish Critical Limits for Each CCP (SEE PRINCIPLE 3)
(30) Critical limits must be specified and validated if possible for each Critical Control Point. In some cases more than one critical limit will be elaborated at a particular step. Criteria often used include measurements of temperature, time, moisture level, pH, Aw, available chlorine, and sensory parameters such as visual appearance and texture.
(31) When the critical limits for a critical control point have been met, the process and/or product is deemed to be safe at that point in the process because the product outcomes have been met. Consequently, where critical limits are exceeded, then the process or product may be deemed to be unsafe.
(32) The critical limits must be measurable, achievable and appropriate to the CCP and hazard(s) being controlled and wherever possible, there should be a scientific basis for the control process and the limits set for each CCP. This information may be found in scientific publications, challenge studies (these must be properly designed to show the destruction, elimination or control of the hazard concerned) and government regulatory agency standards and guidelines. Validation of critical limits proves product outcomes are achieved.
(33) It is important when setting critical control point limits, the variability of any monitoring equipment or process should be considered. The rationale for selected critical limits should be documented.
(34) Once the critical limits have been determined they should be proven (“validated”). This involves the scientific activity/data that demonstrates that the specific hazard(s) at the CCP is eliminated or reduced to an acceptable level, i.e. compliant with product outcomes. Establish a Monitoring System for Each CCP (SEE PRINCIPLE 4)
(35) Monitoring is the scheduled measurement or observation of a CCP relative to its critical limits. The monitoring procedures must be able to detect loss of control at the CCP. Further, monitoring should ideally provide this information in time to make adjustments to ensure control of the process to prevent violating the critical limits. Where possible, process adjustments should be made when monitoring results indicate a trend towards loss of control at a CCP. The adjustments should be taken before a deviation occurs. Data derived from monitoring must be evaluated by a designated person with knowledge and authority to carry out corrective actions when indicated. If monitoring is not continuous, then the amount or frequency of monitoring must be sufficient to guarantee the CCP is in control. Most monitoring procedures for CCPs will need to be done rapidly because they relate to on-line processes and there will not be time for lengthy analytical testing. Physical and chemical measurements are often preferred to microbiological testing because they may be done rapidly and can often indicate the microbiological control of the product. All records and documents associated with monitoring CCPs must be signed by the person(s) doing the monitoring and by a responsible reviewing official(s) of the company.
(36) Monitoring procedures should provide information on:
- (a) who will undertake the monitoring (this person must be trained and have appropriate responsibility to initiate corrective action, or a computer with appropriate recording and software controls);
- (b) frequency of the monitoring including statistically valid sampling regimes;
- (c) what will be monitored;
- (d) where monitoring will occur; and
- (e) how critical limits will be monitored.
Establish (CPP) Corrective Actions (SEE PRINCIPLE 5)
(37) Specific corrective actions must be developed for each CCP in the HACCP system in order to deal with deviations when they occur. The actions must ensure that the CCP has been brought under control. Actions taken must also include proper disposition of the affected product. Deviation and product disposition procedures must be documented in the HACCP record keeping.
(38) Where the critical limits for a CCP have been exceeded, the following corrective actions must be taken:
(39) Bring the defective process back under control.
(40) Determine and control any affected product. All product processed back to the point where the CCP was known to be within limits must be considered “affected” and be treated in accordance with “Management of Non-conforming Dairy Material and Dairy Product” and “Reporting Requirements”. (Applicable to dairy industry trainees)
(41) Take action to ensure the non-conformance does not recur. In this regard the investigation should determine the root cause of the problem, take action to prevent recurrence and follow up with monitoring and reassessment to ensure the corrective action is effective. This step may involve reassessment of the control measures and/or modification of the HACCP Plan.
(42) Corrective action responsibilities should be defined in the HACCP Plan, and recorded. Establish Verification Procedures (SEE PRINCIPLE 6)
(43) Establish procedures for verification. Verification and auditing methods, procedures and tests, including random sampling and analysis, can be used to determine if the HACCP system is working correctly. The frequency of verification should be sufficient to confirm that the HACCP system is working effectively.
Examples of verification activities include:
- (a) Review of the HACCP system and its records;
- (b) Review of deviations and product dispositions;
- (c) Confirmation that CCPs are kept under control.
(44) Where possible, validation activities should include actions which confirm the efficacy of all elements of the HACCP Plan.
(45) The internal verification procedures should detail who is to undertake the verification process(es), the frequency of verification, including sampling regimes, what is to be verified and how verification is undertaken. Hazard Identification and Analysis or HACCP Plan Validation
(46) The Hazard Identification and Analysis or HACCP Plan is validated at least when it Is first developed and following revision, by a competent, internal or external validator on behalf of the company/operator.
(47) Validation involves obtaining evidence that all steps of the Hazard Identification and Analysis or HACCP Plan are effective in achieving the product outcomes.
The Hazard Identification and Analysis or HACCP Plan validation includes:
- (a) review of the scope, product description, intended use;
- (b) review of the process flow and verification;
- (c) review of the hazard identification and analysis;
- (d) confirmation the control measure(s) and critical control points eliminate or reduce the hazard(s) to an acceptable level (product outcomes);
- (e) review of CCP determination;
- (f) review of justification of critical limits, including validation information;
- (g) determination of the ability for equipment to deliver the parameters of the critical limit (e.g. heat treatment in accordance with Animal Products (Dairy) Approved Criteria for the Manufacturing of Dairy Material and Products “Dairy Heat Treatments” which may include crack tests, divert checks);
- (h) determination of whether monitoring activities, corrective action, record keeping and verification activities are appropriate and adequate for the defined hazard and relative to product outcomes. (Dairy elements are specific to dairy industry trainees) (NB. CCP monitoring and CCP corrective action apply to a HACCP Plan only.) HACCP System Audits (Internal Only)
(48) HACCP system audits should review the actual practices and application of any procedures written in the Hazard Identification and Analysis or HACCP Plans.
HACCP system audits may include on-site observations to confirm that events are occurring. These may include confirmation that:
- (a) product description and process flow diagram continue to be accurate;
- (b) monitoring required by the HACCP Plan at the CCPs is performed;
- (c) processes are operating within established critical limits;
- (d) where monitoring has indicated a deviation from critical limits, affected product has been controlled as established and corrective actions have been followed;
- (e) records are filled out accurately.
(49) The audits may cover the entire Hazard Identification and Analysis or HACCP Plans or selected parts. However a full review is recommended periodically to ensure that the Hazard Identification and Analysis or HACCP Plans continues to meet expected outcomes and remains suitable. Where possible, reviews should be carried out under a formal audit procedure with appropriate follow-up for non- conformances to the Hazard Identification and Analysis or HACCP Plans.
(50) Additionally, a review of the HACCP system should occur when changes that may impact on the Hazard Identification and Analysis or HACCP Plans occur.
Examples of changes include:
- (a) introduction of a new raw material;
- (b) changes to the formulation, processing or packing methods and/or system;
- (c) a change to the intended product use;
- (d) a significant food safety event, e.g. pathogen or foreign matter contamination.
(51) In addition, a review of the Hazard Identification and Analysis or HACCP Plans may be undertaken following customer complaints. Equipment Calibration
(52) The calibration of CCP process monitoring instruments needs to be:
- (a) at a frequency to assure continuous accuracy;
- (b) according to procedures established in the HACCP Plan;
- (c) against a recognised standard.
(53) When equipment monitoring a CCP is out of calibration, the CCP is considered to have been out of control since the last documented calibration. Product Sampling and Testing
(54) The product sampling and testing regime that is used to verify the product outcomes of the HACCP Plan have been met, shall be recorded, validated and available for audit. Guidance for establishing the sampling and testing regime is available in the MPI HACCP Plans operational guideline located on the MPI Food Safety website
Establish Documentation and Record-Keeping (SEE PRINCIPLE 7)
(55) Efficient and accurate record-keeping is essential to the application of a HACCP system. HACCP procedures should be documented. Documentation and record-keeping should be appropriate to the nature and size of the operation.
(56) Documentation examples are:
- (a) Hazard analysis;
- (b) CCP determination;
- (c) Critical limit determination;
- (d) Record examples are;
- (e) CCP monitoring activities;
- (f) Deviations and associated corrective actions;
- (g) Modifications to the HACCP system.
(57) Records are essential for reviewing the adequacy of the HACCP Plan and the compliance of the HACCP system to the plan. As a minimum the following documentation should be kept:
- (a) HACCP Plan and the support documentation used to develop the Plan e.g. data used to establish the adequacy of the critical limits in ensuring the safety of the product or data used to establish sampling and testing rates.
- (b) hazard identification and analysis data;
- (c) CCP determination process;
- (d) CCP monitoring records e.g. temperature, time etc;
- (e) Corrective Action Records (including product disposition records);
- (f) verification activity records e.g. sampling and testing regimes;
- (g) verification records e.g. audit reports, etc.
(58) Documentation and record keeping should be undertaken in accordance with the requirements Implementation
(59) There are a number of ways that a Hazard Identification and Analysis or HACCP Plan can be implemented. This will depend on the size and complexity of the operation and resources available. The company must decide the best way to introduce the Plan to the workplace.
(60) For the Hazard Identification and Analysis or HACCP Plan to be successful, it should be effectively implemented. The first stage of effective implementation is to ensure that effective training has been undertaken.
(61) Training of personnel in industry, government and academia in HACCP principles and applications, and increasing awareness of consumers are essential elements for the effective implementation of HACCP. As an aid in developing specific training to support a HACCP Plan, working instructions and procedures should be developed which define the tasks of the operating personnel to be stationed at each Critical Control Point.
(62) Cooperation between primary producer, industry, trade groups, consumer organizations, and responsible authorities is of vital importance. Opportunities should be provided for the joint training of industry and control authorities to encourage and maintain a continuous dialogue and create a climate of understanding in the practical application of HACCP.
(63) Then the following points are recommended:
- (a) external assessment of the Hazard Identification and Analysis or HACCP Plans.
- (b) evaluation of Hazard Identification and Analysis or HACCP Plans
(64) The validated Hazard Identification and Analysis or HACCP Plan is evaluated by the recognized agency when it is first developed and following all significant changes.
Some of the Companies & Brands Food Safe works with

Here’s how Food Safe Collaborates with Food Companies:
For more information, call us on +64 9 2814226 or email [email protected]
Food Safe Ltd is Accredited by the New Zealand Government + is a Category 1 NZQA-Registered PTE. Training complies with Codex Guidelines
Our training is trusted by both well known New Zealand and Global food companies and heaps of small teams too!
Our training is science-based on New Zealand regulatory and globally recognised best practice including – MPI, FAO, FDA, EU
Food Safe’s advisory committee includes leading experts, quality and compliance managers, and governance experts. For even more information about Food Safe and the companies we work with, click here
Food Safe’s Training:
- Complements compliance requirements
- Is simplified and visual, and supportive of implementing learning back on-job
- Is delivered by a trained ISO 9001 & 22000 lead auditor
- It is delivered by a trainer with first-hand knowledge and experience in high
- compliance operations where Food Safe also operates, such as the meat, dairy, and seafood sector. This allows us to transfer best practice.
For more information, call us on +64 9 2814226 or email [email protected]




